In the quiet corners of biotechnology, where the boundaries between science fiction and reality blur, Andrew Hessel stands as a quiet architect of tomorrow. With one foot in the verdant wine country of California and another in the crisp air of Canada, he embodies the dual nature of his field: rooted in the natural world yet propelled by human ingenuity. Andrew, a pioneer in synthetic biology, speaks with the measured confidence of someone who has witnessed the decoding of humanity’s genetic blueprint and now advocates for rewriting it.
His conversations reveal not just the mechanics of life but the profound implications for our species, our economy, and our investments in the future.
Consider the Human Genome Project, that monumental endeavor launched in 1990 to map the three billion base pairs that make us who we are. Andrew was there on the corporate front lines, collaborating with visionaries like Craig Venter to race against academic teams in a quest that ended in a triumphant tie for all humankind. The project, completed in 2003, unlocked diagnostics and deepened our grasp of health and disease. Yet Andrew’s gaze extends beyond reading the code; he co-founded the Genome Project-write in 2016, an initiative to synthesize entire genomes. This shift from reading to writing promises corrections for genetic ailments in the near term and, philosophically, the reshaping of human potential over generations.
Such advancements do not occur in a vacuum. Biotechnology follows its own relentless pace, akin to the semiconductor industry’s famous trajectory but often surpassing it. The Carlson curve, named after biologist Rob Carlson, charts the plummeting costs and soaring capabilities of DNA sequencing and synthesis. While Moore’s law doubles computing power roughly every two years, sequencing technologies have accelerated up to five times faster at points, driven by innovations that make genetic data as accessible as digital files.
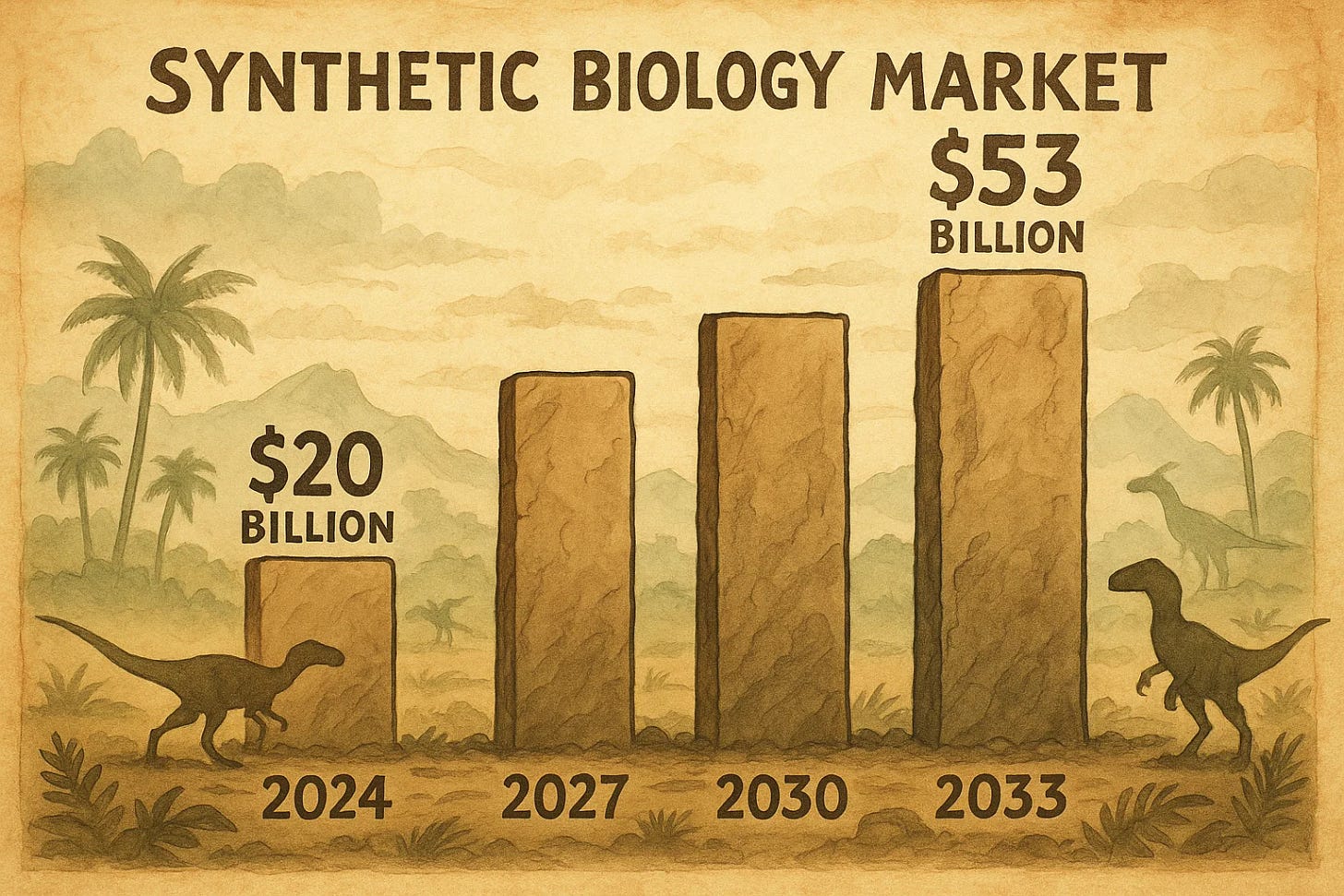
Investors take note: this curve underpins a market projected to grow from nearly $20 billion in 2024 to over $53 billion by 2033, fueled by applications in medicine, agriculture, and materials. Companies riding this wave, such as those specializing in gene editing, attract venture capital in the billions, promising returns that echo the tech booms of decades past.
Andrew’s insights illuminate how artificial intelligence amplifies this momentum. AI now deciphers the arcane language of A’s, T’s, G’s, and C’s, designing proteins and even entire organisms from scratch. Only a few months ago, systems successfully created a bacteriophage, a virus specifically designed to target bacteria, marking a significant milestone in the creation of life in machines. Proteins, the workhorses of biology, number in the billions in nature, but AI explores trillions more possibilities, opening doors to novel drugs and materials.
This convergence is not mere hype; it represents a paradigm where biology becomes programmable, much like software. For financiers, the implications are vast: synthetic biology startups secured over $10 billion in funding in 2024 alone, with genome editing sectors expected to expand at nearly 29 percent annually through 2032.
To see this fusion in action, watch this video exploring a synthetic DNA factory where automation and AI engineer new life forms: This Synthetic DNA Factory Is Building New Forms of Life.
Venturing further into the audacious, Andrew points to Colossal Biosciences, a company blending AI with genomics to revive extinct species. They are not cloning woolly mammoths outright but editing Asian elephant genomes to imbue them with mammoth-like traits: cold resistance, shaggy fur, and curved tusks. This high-volume genetic tinkering relies on fragmented ancient DNA assembled computationally, pushing the limits of synthesis technologies. Colossal’s work, while captivating, serves a deeper purpose: mastering these tools ensures no species need vanish forever, provided we preserve their code. Investment in such ventures is booming; Colossal itself raised $200 million in January 2025, followed by another $120 million in September, catapulting its valuation to $10.3 billion and drawing backers like Peter Jackson and prominent tech funds. This not only highlights the allure of de-extinction but also underscores biotechnology’s role in biodiversity markets, potentially worth trillions in ecosystem restoration and conservation economies.
The ethical undercurrents run deep. Andrew envisions a future where we “de-extinct” ourselves through cryogenic preservation and cloning, enhanced by edits to eradicate diseases like cancer. Drawing parallels to in vitro fertilization, which has birthed millions, he anticipates cloning’s normalization, augmented by AI-driven tweaks for healthier iterations. This sci-fi-adjacent prospect raises questions of identity and equity, yet it also beckons investors toward longevity markets, where anti-aging and personalized medicine could redefine trillion-dollar industries.
For a glimpse into the science of reviving species like the dodo, view this breakthrough update from Colossal Biosciences: BREAKTHROUGH: Bringing the Dodo Back from Extinction.
Inspiring the next wave of innovators is crucial, Andrew argues. Programs like the iGEM competition gather thousands of young synthetic biologists annually, fostering projects that tackle global challenges. Yet scalability remains a hurdle; hands-on kits and community labs exist, but Andrew champions cloud-based robotic facilities, where students design experiments virtually and execute them remotely. This process democratizes access, mirroring how personal computers unleashed coding creativity. Gamification, akin to Minecraft communities, could accelerate engagement, especially among borderless Gen Z cohorts who prioritize planetary collaboration over national silos.
Investment here ties into decentralized science, or DeSci, where blockchain enables transparent funding and token-based economies for research. Projects like VitaDAO and Molecule exemplify this, raising millions through crypto to back longevity and drug discovery. By sidestepping paywalled journals, DeSci invites societal input, potentially unlocking breakthroughs faster than traditional models. Venture firms are pouring in, with DeSci platforms attracting over $500 million in 2025, blending biotech with web3 for hybrid returns.
Peering three to five years ahead, absent regulatory or fiscal barriers, Andrew foresees a world where single-celled organisms are engineered routinely, producing foods, medicines, and materials at scale. This “personal computer of life” unlocks godlike capabilities, sustaining humanity through millennia. Financially, it heralds a bioeconomy rivaling tech giants, with synthetic biology poised to disrupt supply chains and create new asset classes.
Finally, look at the original Human Genome Project’s epic journey in this documentary-style overview: The Human Genome Project: The 13-Year Quest to Chart the Mysteries of Human Genetics.
Andrew’s message is clear: biology’s programmability is inevitable. Whether for personal health, business innovation, or familial legacies, understanding and investing in it now could yield dividends beyond measure. As he invites connections on LinkedIn or Substack, one senses the dawn of an era where life itself becomes our greatest canvas.